TACKLE INFECTIOUS DISEASES AND ANTIMICROBIAL RESISTANCE
GET CLINICALLY ACTIONABLE INFORMATION FROM MICROBIAL DNA
POWERFUL SOLUTIONS FOR CLINICIANS AND RESEARCHERS
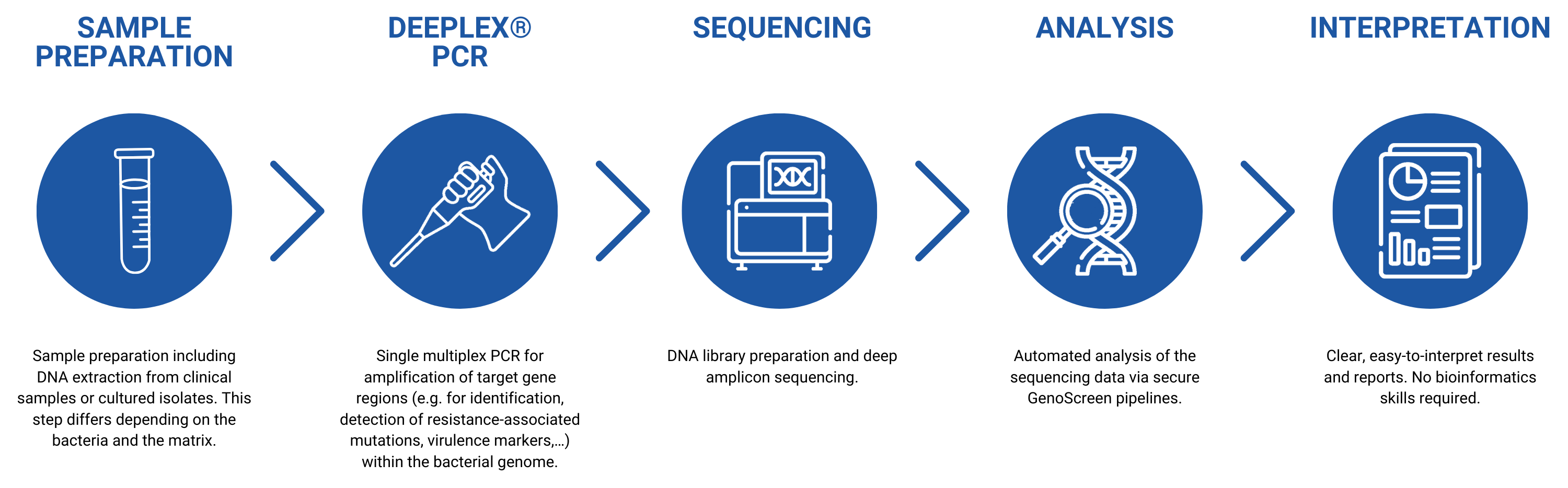
The Deeplex® engineering is a next-generation sequencing based technology, developed by GenoScreen, for accurate characterization of specific pathogens and prediction of their antimicrobial resistance.
This technology provides clinicians and researchers with extensive information to tackle major infectious diseases worldwide.
FAST. SECURE. EASY TO INTERPRET.
Welcome to the future of diagnosis
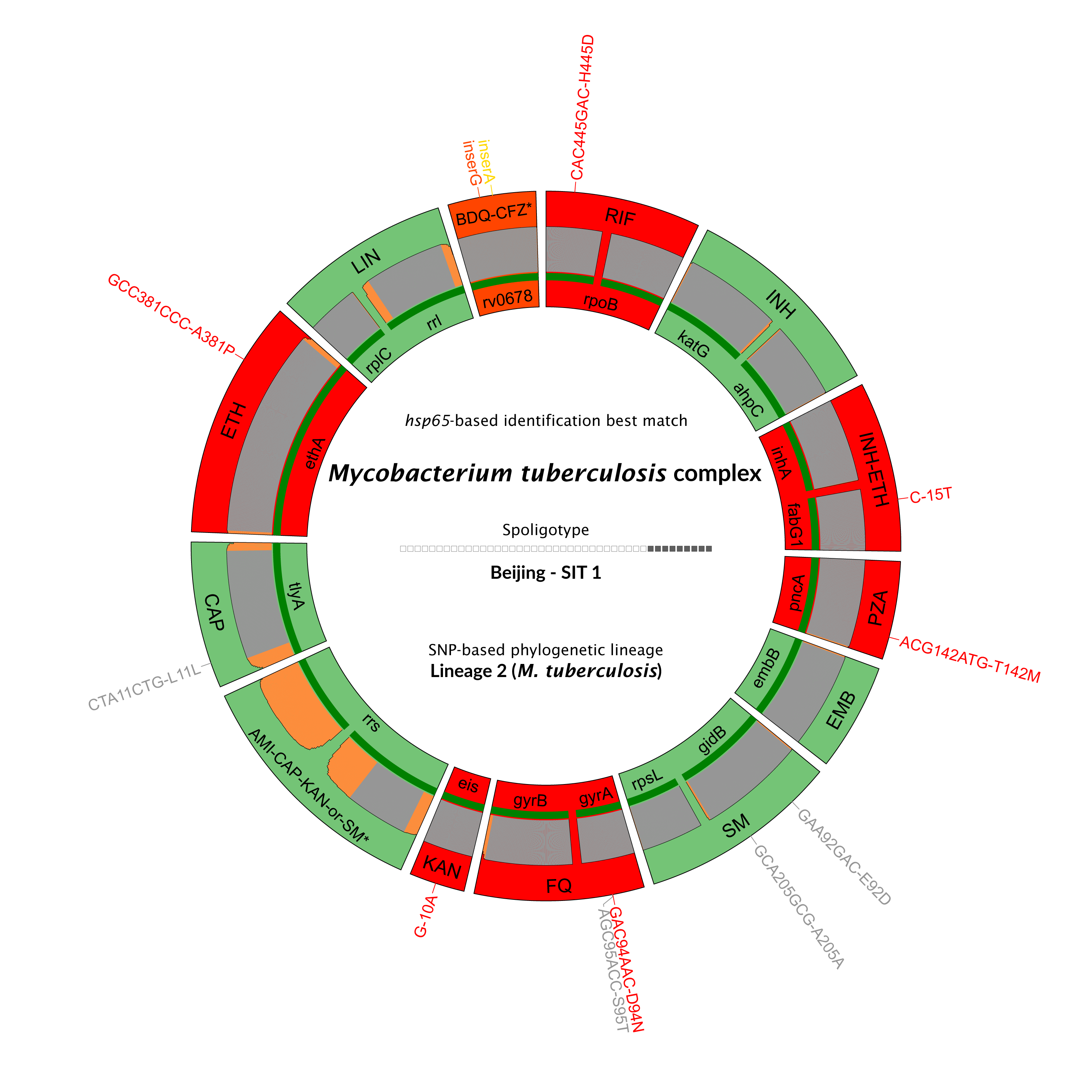
Information on bacterial identification and strain genotype
Gene or genomic region targeted by Deeplex® amplification
Drug associated with the gene target
Identified mutations are noticed in red if associated with antimicrobial resistance
Limit of detection and target coverage
Key results at first glance.
Get a clear overview of each sample, the Deeplex® map visually links antibiotics to genomic regions for rapid and straightforward interpretation.
All detected mutations are mapped for precise identification and extensive prediction of resistance profiles.
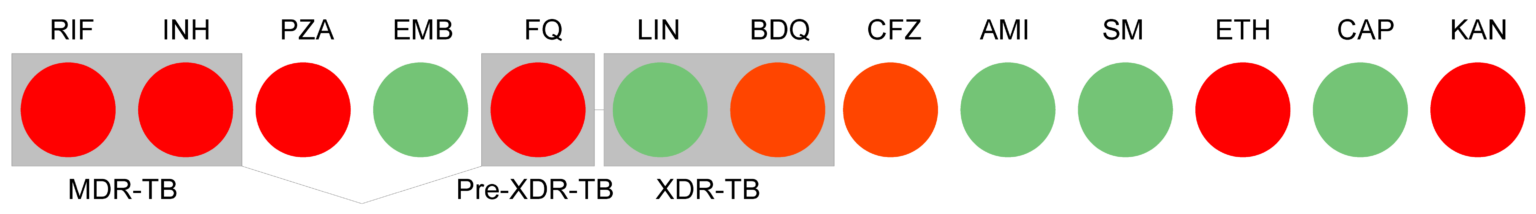
Resistotype: summary of antibiotic resistance prediction
Example of a Deeplex® Myc-TB map and its associated resistotype. Green: No sequence variants identified or variants known not to be linked with resistance. Red: Identification of one or several variants associated with resistance. The Deeplex® map is a registered design.
A streamlined workflow